-
Excitation wavelength dependence of the charge separation pathways in tetraphorphyrin-naphthalene diimide pentads
D. Villamaina, M. Kelson, S. Bhosale and E. Vauthey
Physical Chemistry Chemical Physics, 16 (11) (2014), p5188-5200


DOI:10.1039/c3cp54871f | unige:34401 | Abstract | Article HTML | Article PDF

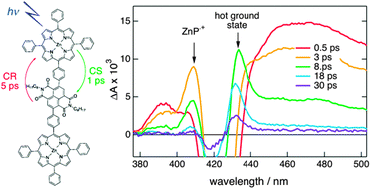
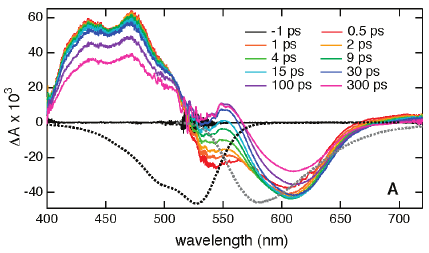
The excited-state dynamics of two multichromophoric arrays composed of a naphthalene diimide centre and four zinc or free-base porphyrins substituted on the naphthalene core via aniline bridges has been investigated using a combination of stationary and ultrafast spectroscopies. These pentads act as efficient antennae as they absorb over the whole visible region, with a band around 700 nm, associated with a transition to the S1 state delocalised over the whole arrays, and bands at higher energy due to transitions centred on the porphyrins. In non-polar solvents, population of these porphyrin states is followed by sub-picosecond internal conversion to the S1 state. The existence of a charge-separated state located above the S1 state could enhance this process. The decay of the S1 state is dominated by non-radiative deactivation on the 100 ps timescale, most probably favoured by the small S1-S0 energy gap and the very high density of vibrational states of these very large chromophores. In polar solvents, the charge-separated state lies just below the S1 state. It can be populated within a few picoseconds by a thermally-activated hole transfer from the S1 state as well as via sub-picosecond non-equilibrium electron transfer from vibrationally hot porphyrin excited states. Because of the small energy gap between the charge-separated state and the ground state, charge recombination is almost barrierless and occurs within a few picoseconds. Despite their very different driving forces, charge separation and recombination occur on similar timescales. This is explained by the electronic coupling that differs considerably for both processes.